Have you ever wondered why ice sticks stubbornly to airplane wings, why water droplets cling to your car windshield, or why marine organisms attach themselves to ship hulls? These seemingly simple phenomena have enormous implications for safety, energy efficiency, and environmental sustainability. Imagine if we could design surfaces that repel water, ice, and dirt—surfaces that stay clean, safe, and functional without constant maintenance.
This is where Slippery Covalently Attached Liquid Surfaces (SCALS) come in. They are a fascinating new class of materials that could transform industries ranging from aviation and energy to healthcare and consumer products. Our latest review published today Nature Protocols (link here) explains how SCALS are defined, how they work, and how to make them. The review is led by Dr Isaac Gresham and brings together three of the international leading groups in the area of SCALS: the Chiara Neto group at the University of Sydney, the Glen McHale/GARY WELLS group at the The University of Edinburgh and the Kevin Golovin group at the University of Toronto, for what I like to call “the mother of all reviews”.
What Are SCALS?
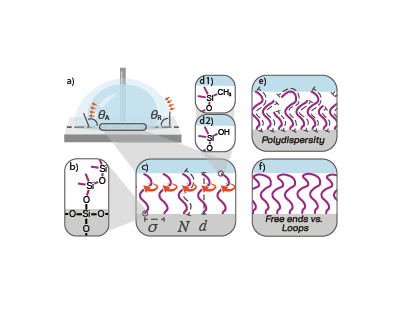
SCALS—sometimes called quasi-liquid surfaces or liquid-like surfaces—are ultra-thin layers of polymers or oligomers that remain liquid at room temperature. These layers are covalently attached to a solid substrate, meaning they are chemically bonded and won’t simply wash away.
Think of SCALS as creating a “liquid skin” on a solid surface. This skin is so smooth and slippery that water droplets, ice crystals, and even biological contaminants struggle to stick. The result? Surfaces that shed droplets effortlessly, resist icing, and prevent fouling. Beyond practical uses, SCALS also serve as model systems for fundamental science, helping us understand wetting, evaporation, and self-assembly at the molecular level.
At first glance, SCALS seem straightforward: attach a liquid-like polymer to a surface, and you’re done. But here’s the catch—the apparent simplicity is misleading. Achieving reproducible results requires precise control of reaction conditions, to achieve the required complete coverage, chain length, and grafting density. Small variations in humidity or surface cleanliness can dramatically change the outcome.
Our review reproduced six prominent synthetic methods in our three laboratories across the globe, compared their strengths and weaknesses, and identified the key parameters that determine success.
Our review includes:
– Step-by-step procedures for preparing the most common SCALS system: polydimethylsiloxane (PDMS) bound to silica surfaces via silane chemistry.
– Comparative analysis of synthetic methods, highlighting their advantages and limitations.
– Optimization guidelines for reproducibility
– Characterization techniques to verify that the surface you’ve created truly behaves like a SCALS.
Comparing methods for preparing slippery liquid-like polydimethylsiloxane coatingstrebuchet.public.…